Ever wonder how we get a ‘new’ flu vaccine each year? For decades, there has been a systematic and step-by-step approach to making a new flu shot for each season. On February 27, the first step of this process, a critical meeting of the advisory committee that decides what’s included in the flu shot, was canceled with no plans to reschedule.
That’s not the only vaccine meeting that was canceled. The ACIP (The Advisory Committee on Immunization Practices) was scheduled to meet from Feb 26-28 to discuss and vote on important immunizations and practices such as: influenza, chikungunya, COVID-19, RSV, Mpox, HPV, Pnemococcal, CMV, and Lyme disease (Draft schedule here). Normally, the public is allowed to submit comments prior to this meeting, through a federal portal which was suppposed to be available from Feb 3-17th. This portal, however, was absent (presumably because the Trump White House ordered all forward facing CDC portals to be frozen at that time). HHS issued a statement that the ACIP meeting would be postponed to allow for public comments and rescheduled at a future date. The portal is currently active, but the ACIP meeting date says “TBA”.
The American College of Physicians issued this statement:
“Our country is currently facing the worst epidemic of influenza in several decades, a measles outbreak in Texas, and an ongoing national outbreak of pertussis. The CDC must promptly reschedule this critical meeting. We call on Secretary Kennedy and other officials to ensure that the advice of epidemiologists, researchers, physicians, and other experts in disease and immunizations remains primary in helping to ensure the public's health."

So, here is the normal process of making the yearly flu shot. Since we no longer live in normal times, I’m not sure what is going to happen.
Surveillance
This is Year-Round. The WHO runs the Global Influenza Surveillance and Response System (GISRS) which collects data from around the world in real-time, provides public flu tracking data, monitors new emerging strains and issues alerts. They also make recommendations for what should be included in the flu vaccines for the Northern Hemisphere (in Feb) and the Southern Hemisphere (in Sept).
There are 152 National Influenza Centers in over 114 countries around the world. They collect virus samples from their home countries and do preliminary analysis (what species and strain of flu, etc) and then send samples to the WHO for more advanced genetic testing. America’s only site is at the CDC. (List of sites).
Within GISRS there are 7 National Collaboration Centers (NCCs) and 4 Essential Regulatory Labs (ERLs). The NCCs do research on flu and share that with the WHO, as well as serve as a distribution point for reference vaccine viruses. The ESLs provide needed reagents for flu vaccine production, and vaccine guidance.
The CDC compiles it’s own flu data in the Flu Tracker, which is updated weekly. Here is the current flu info for America.
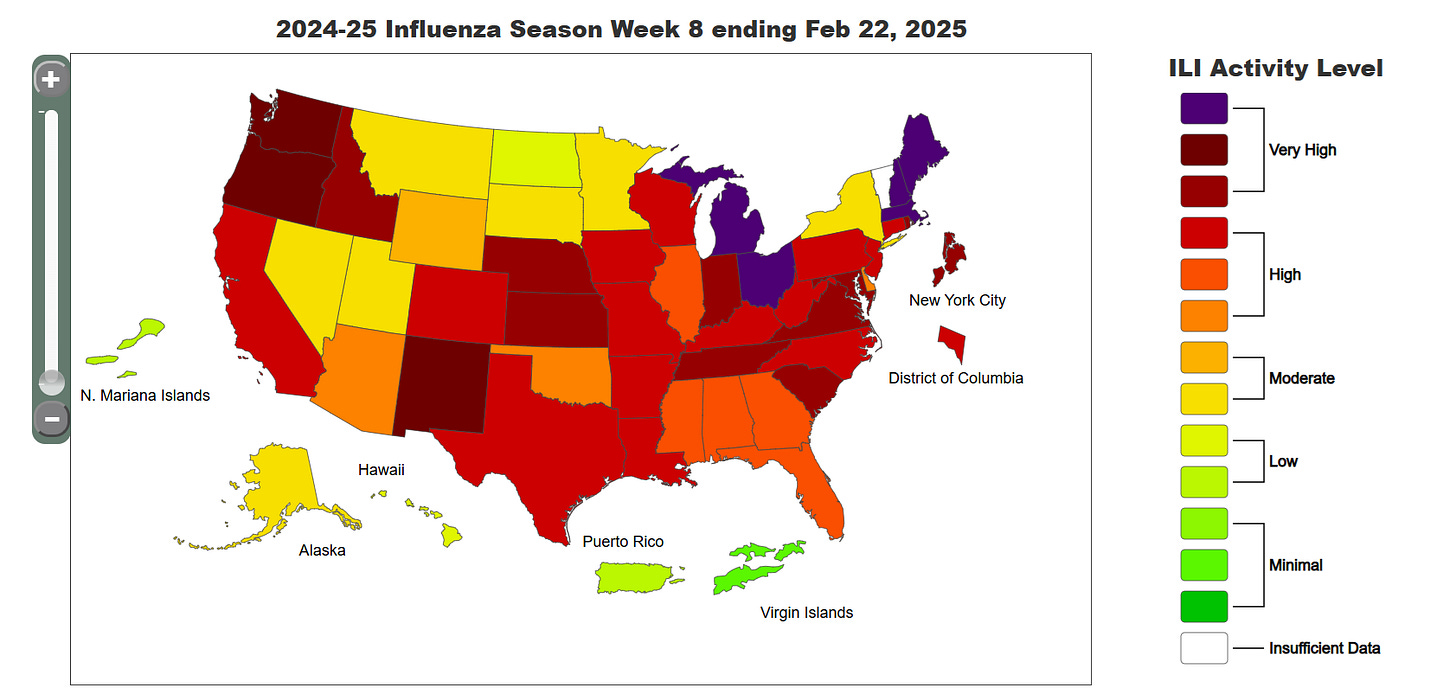
While it looks like flu season may have peaked, so far this season: 21,000 Americans have died of influenza infection, including 98 children.
Step 1: Strain selection
In March, the Vaccine and Related Biological Products Advisory Committee (VRBPAC) is supposed to meet to decide which of the flu strains should be included in the upcoming season’s shot. The viruses that are chosen are sent to the flu vaccine manufacturers to begin vaccine production. The ACIP also meets monthly to create the recommendations for flu vaccine distribution and uptake (like which vaccine is appropriate for kids, the elderly, travelers, etc and this info is sent to clinicians for reference).
These are the two meetings that have been canceled.
So, what is going to happen? Honestly, it’s impossible to tell since this has never happened once in the history of American flu vaccine manufacturing. A spokesman for HHS said: “The FDA will make public its recommendations to manufacturers in time for updated vaccines to be available for the 2025-2026 influenza season” so it looks like the FDA might make recommendations without the advice of the VRBPAC committee… which is unprecedented. The VRBPAC meeting is publicly available and they also do a “post-mortem” on last year’s flu vaccine to see where improvements could be made. It’s unclear if any of that will happen now. Will the FDA keep this process transparent? Who knows. Who at the FDA is making the vaccine recommendations? Who knows.
It’s also possible that we can just use the WHO recommendations for the Northern Hemisphere, which may not be as tailored to America as our own recommendations would have been. Paul Offit, a member of VRBPAC, said about downloading WHO recommendations: “Yeah, basically. It’s not ideal, but what else are the vaccine manufacturers going to do?”
Step 2: Manufacturing
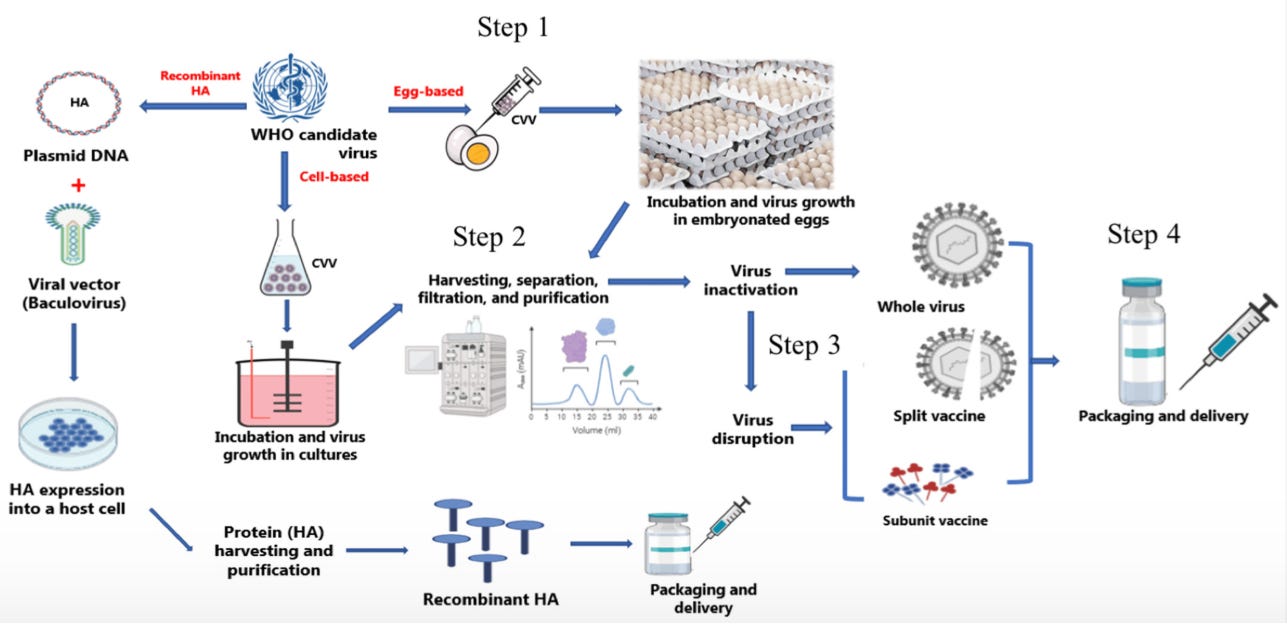
The selected strains are sent to the various vaccine manufacturers. Many times, the vaccine manufacturers try to make their own predictions about which strains will be selected so that they can get a head start. They then make flu vaccine using the selected strains. There are three main ways to make a flu vaccine (I’ll use 2024 as a reference):
Grow the flu virus in embryonated chicken eggs. 7/9 of the vaccines are made this way.
Grow the flu virus in cell culture (cells in petri dishes). 1/9 of the vaccines.
Make recombinant flu proteins (purified proteins made in a controlled system. No actual flu VIRUSES are made, just the proteins). 1/9 of the vaccines.
Ok, y’all. Will the bird flu and egg shortages impact flu vaccine production? Not likely. The eggs used for vaccine production are not part of the food supply (they are embryonated, they have little chick babies inside, unlike the unfertilized eggs we eat). Vaccine manufacturers keep their hens very isolated so that they cannot be infected by things like bird flu. It is essential that eggs for vaccine production are as clean as possible, so the chickens are isolated. William Schaffner, MD, says that after a bird flu scare 20 years ago, “it became clear that the entire egg production had to be ... protected from wild bird contamination.”
However the flu vaccine virus is made, it is then purified and all the strains are combined into one vial. The manufacturers test the vaccines for purity/sterility and viral concentration, and send those results along with lot samples to the FDA.
Step 3: FDA approval
Technically, all the vaccine manufacturers that make flu shots for America have already received FDA approval. The components of the vaccine (other than the actual virus itself which changes from year to year) and the method of manufacturing have gone through the extensive (and expensive!) years-long FDA approval process in the past. The manufacturers submit a supplement to their license each year for the updated vaccines. For inactivated vaccines (which include viruses that are NOT alive), no new clinical trials are needed. For the one live flu vaccine (the squirt up the nose one), an abbreviated clinical trial of about 300 people needs to be performed for each year before approval is granted by the FDA.
The FDA approves the supplement to the license (and the complete approval for the live vaccine) and starts “lot release” usually in July. Basically this allows certain “lots” or batches of flu vaccine to be released for distribution. Manufacturers then start shipping the vaccine to distribution points.
Step 4: Vaccination and vaccine efficacy
Once vaccines have been shipped, they are available at various places for us (pediatricians offices, grocery stores, drug stores, health departments, etc). Some lots are sent early in the season for high-risk patients like those in nursing homes. Usually, health departments have vaccination campaigns to promote flu vaccines. This year, however, things may be different. Already, Louisiana has banned promotion of flu vaccines. An internal memo stated: “Employees could not send out press releases, give interviews, hold vaccine events, give presentations or create social media posts encouraging the public to get the vaccines. They also could not put up signs at the department's clinics that COVID, flu or mpox vaccines were available on site.”
Vaccine is also tested for how well it works and if it is “matched” to the actual flu strains circulating. Ferrets are vaccinated using the finished flu vaccine and their antibodies are collected (ferrets are one of the experimental animals that are very similar to humans in terms of flu transmission). The actual circulating flu strains are collected at the NICs and tested using the ferret antibodies. These tests showed that out of the 4 strains included in the 2024-2025 flu vaccine, two strains were completely recognized by the ferret antibodies (good), for one strain, 50% were recognized by the antibodies (meh), and for the last strain, no circulating viruses were collected.
This indicates that 2/4 of the chosen vaccine viruses included in the 2024-25 flu vaccine were a good match. Not the best…but still better than nothing.
Circulating viruses are also tested against our anti-viral medications to see if the medicines work. Less than 1% of the circulating flu viruses were resistant to flu medication, so that’s pretty good news.
Where we stand now.
I’m not really sure. Apparantly the FDA is making recommendations for the vaccine without expert advice and without transparency. Some good news: even though Trump banned government employees from working with the WHO, an exemption was made allowing FDA members to participate in the WHO meeting about influenza. The USA occupies 2/10 seats on that board, so our imput is limited, but at least we were at the table.
Since flu vaccine grown in eggs takes 6 months, we are in a time crunch here. Manufacturers can wait for recommendations until the end of March, but no later. So, it remains to be seen how (or who at) the FDA will decide what’s in our flu shots. Theoretically, they could also stop the approval of the supplement to the license, or not approve the “lot release” and prevent manufacturers from shipping flu vaccines this year. However, the government has already placed it’s preorders for next seasons vaccines, and Sanofi (one of the manufacturers) has already begun production, so I think that’s unlikely.
Paul Offit offered another perspective on what FDA is doing though:
“What worries me about this, I think it’s part of the bigger picture, which is this sort of dissolution of public health infrastructure, that we don’t need experts anymore….We don’t need experts advising us at the CDC, we don’t need experts advising us from the FDA. We’ll just figure it out ourselves.”
Stay happy, healthy, vaccinated and informed,
Jessica at TCA
CDC. Advisory Committee on Immunization Practices.
Brown, S. Advisory Committee on Immunization Practices February Meeting Postponed. US News and World Report. Feb 26, 2025.
CDC. MEETING OF THE ADVISORY COMMITTEE ON IMMUNIZATION PRACTICES (ACIP). Draft Schedule.
WHO. Global Influenza Surveillance and Response System.
US FDA. FDA’s Critical Role in Ensuring Safe and Effective Flu Vaccines
Faust, J. Scoop: FDA vaccine meeting abruptly canceled. Substack. Feb 26, 2025.
Robertson, R. Will the Egg Shortage Affect Flu Shots?. MedPage Today. Feb 19, 2025.

